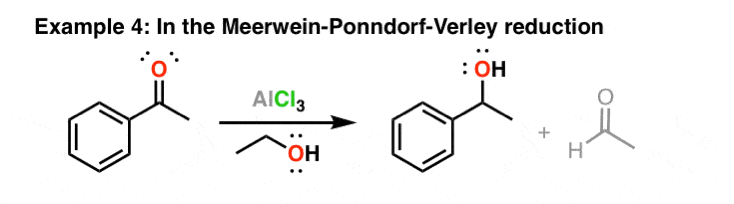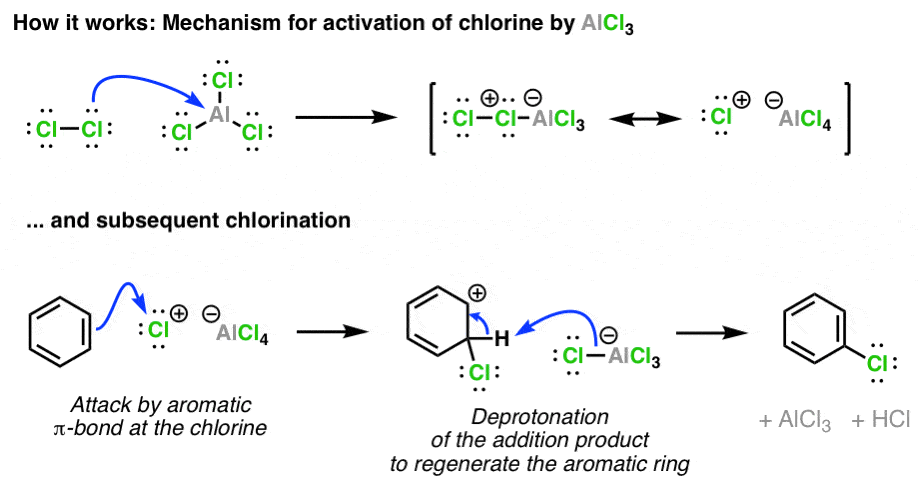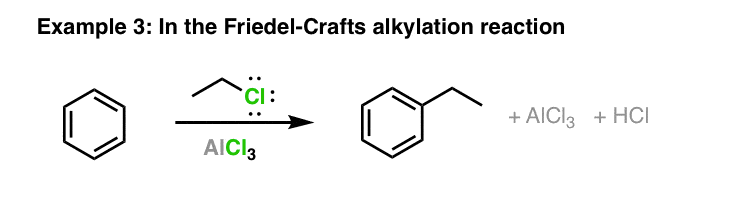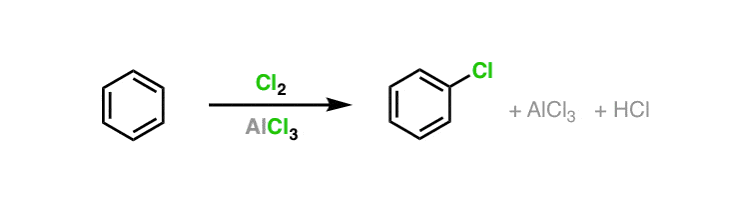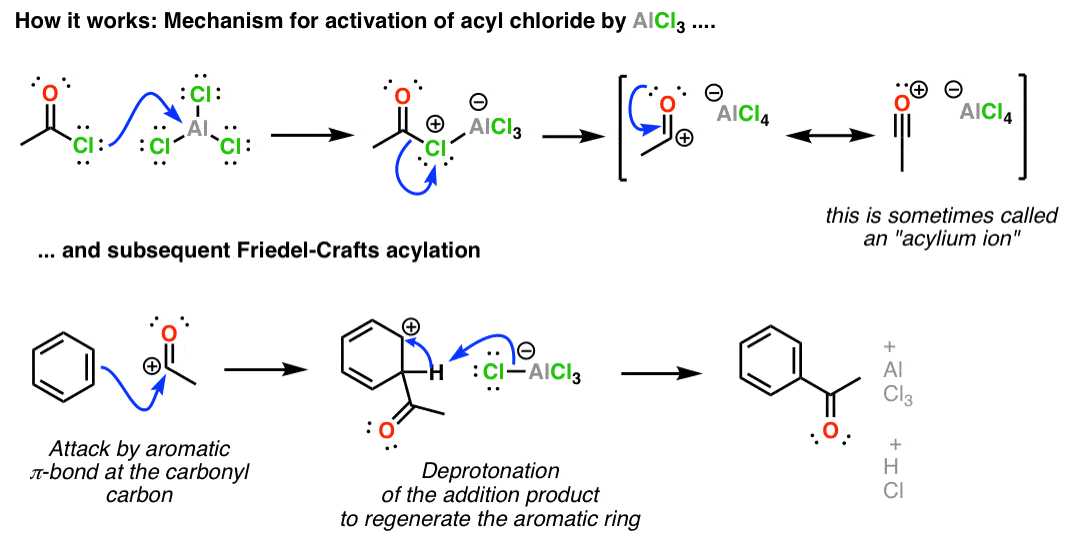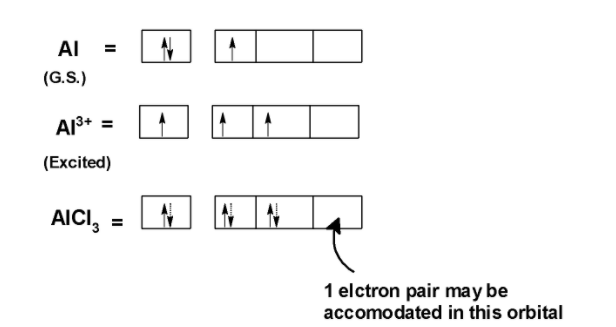
Statement: Aluminium chloride $\\text{ AlC}{{\\text{l}}_{\\text{3}}}\\text{ }$ is a Lewis acid because it can donate the electron.If a given statement is true enter 1 if false enter 0.

Question Video: Identifying the Species That Is Not a Lewis Acid in a Set of Chemical Formulas | Nagwa
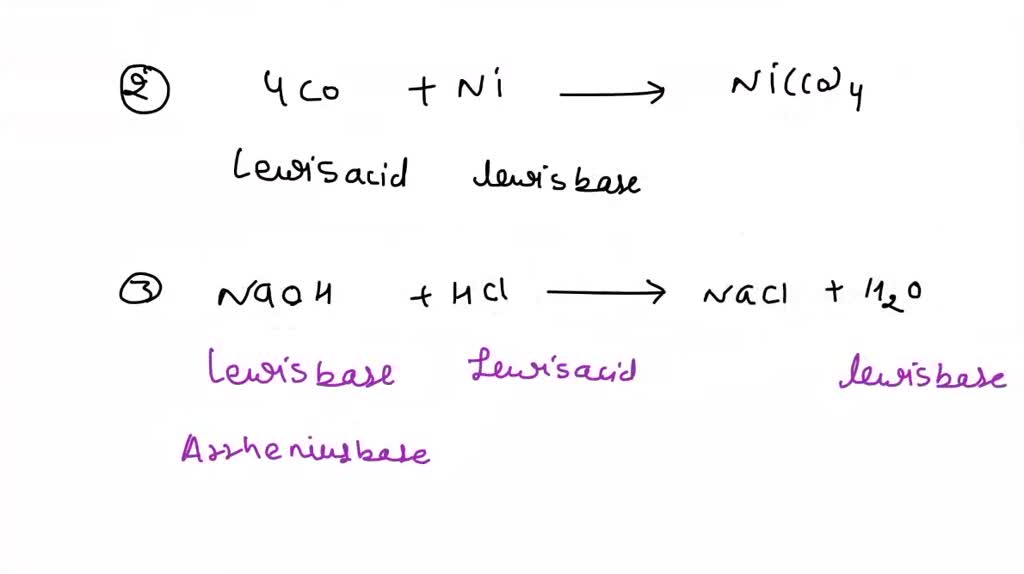
SOLVED: Circle the Lewis Acids and put a box around the Lewis bases. Na+ NH3 BF3 Pt2+ AlCl3 Fe2+ CN- H2O Ag+ H2S H+ CH3COO- Identify the Lewis acid and Lewis base

Draw the Lewis Structure of the product formed from the reaction between ammonia and aluminum chloride. | Homework.Study.com
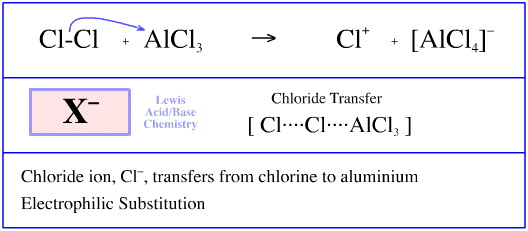
Is the following acid-base reaction Arrhenius, Bronsted-Lowry, or Lewis: AlCl3 + Cl --> AlCl4- | Socratic